Chemistry for Restoration. Painting and Restoration Materials
54,00€ Il prezzo originale era: 54,00€.51,30€Il prezzo attuale è: 51,30€.
Browse the book in preview by sliding the image to the left.
Click on the image you are interested in to enlarge it.
Sfoglia il libro in anteprima facendo scorrere l’immagine a sinistra.
Clicca sull’immagine che ti interessa per ingrandirla.
We consider the production of a specific book, Chemistry for Restoration, addressing all general and applied issues of chemistry, the “alien” scientific discipline primarily involved in conservation, instrumental in collecting and organising the many experiences and advancements that during recent decades have often been accumulated in a disorganised manner. Of course this book is also useful in addressing educational and training needs. Today, schools of conservation and restoration are present all over the world. Young students who choose this type of professional activity should be trained, from the very beginning, within the typical interdisciplinary environment that characterizes the conservation of cultural heritage. This chemistry book dedicated to conservation and restoration aims at communicating and transferring to this field specifically addressed and targeted notions and concepts, without losing sight of a high scientific level…
Authors: Mauro Matteini, Rocco Mazzeo, Arcangelo Moles
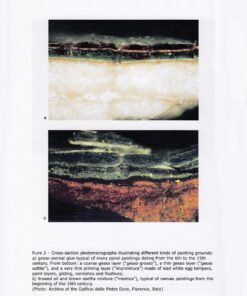
Pages 372, paperback, cm 17×24, illustrations in black and white, 20 color plates, year 2016
Isbn/Ean code: 9788840444505
—————————–
Presentations
Giorgio Bonsanti
Brunetto Giovanni Brunetti
Michel Menu
Foreword
PART I
PAINTING MATERIALS AND PRODUCTS FOR RESTORATION
Chapter one
PIGMENTS
1.1 – Pigments and dyes
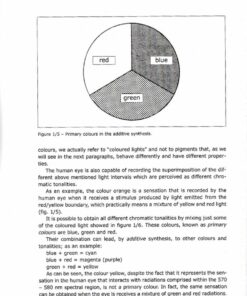
1.2 – Optical properties – The colour
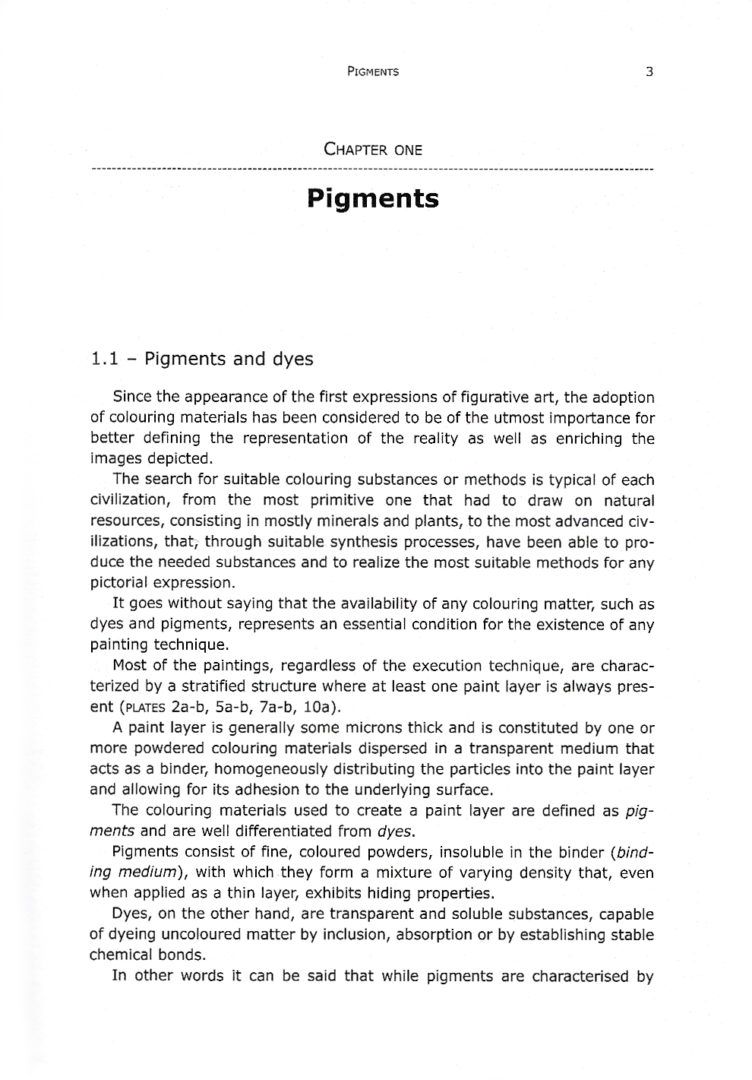
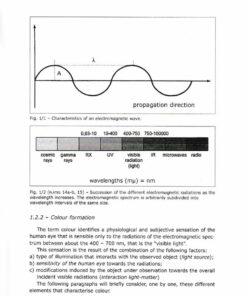
1.2.1 – Electromagnetic radiations and visible light
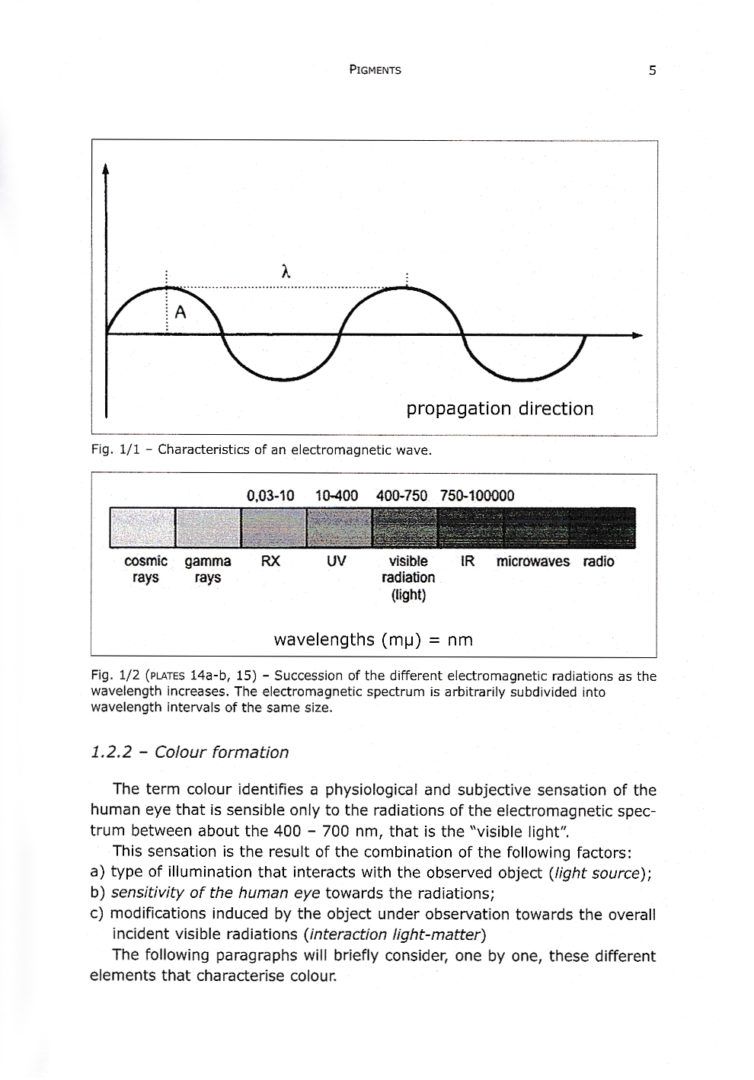
1.2.2 – Colour formation
1.2.3 – Light sources
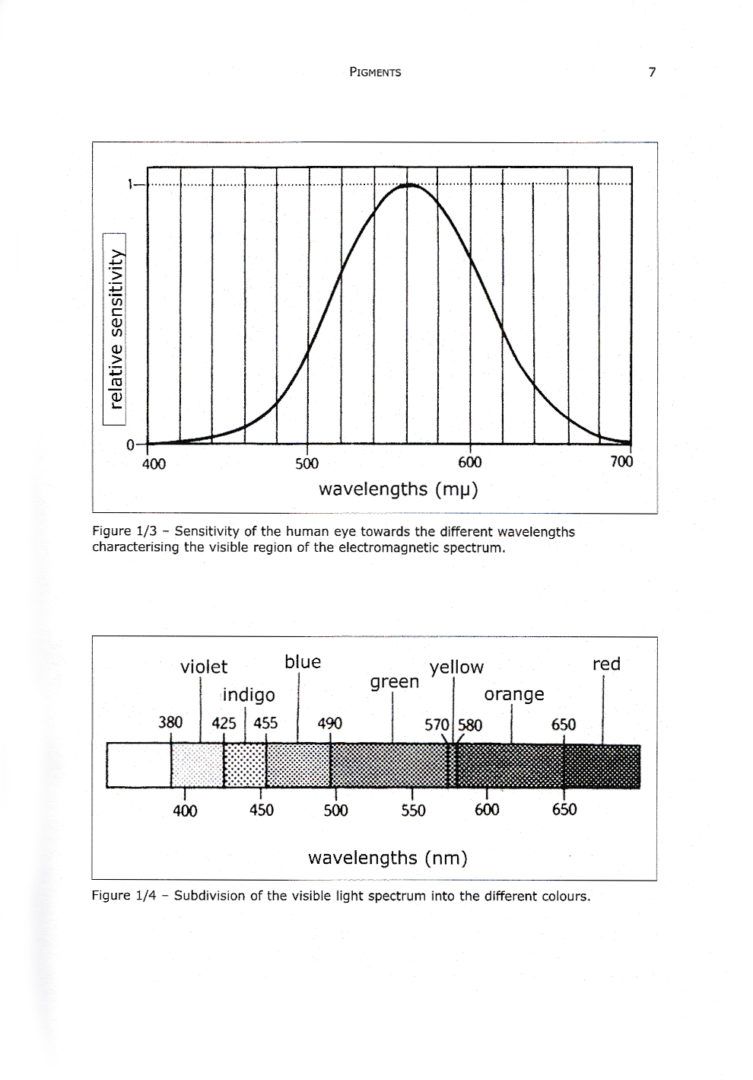
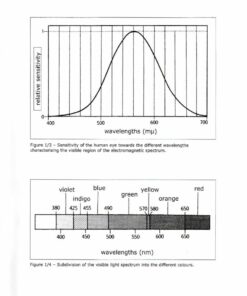
1.2.4 – Sensitivity of the human eye and the perception
of colour as a physiological effect of the eye
1.2.5 – Interaction light/materials
1.2.6 – Light and paint layer
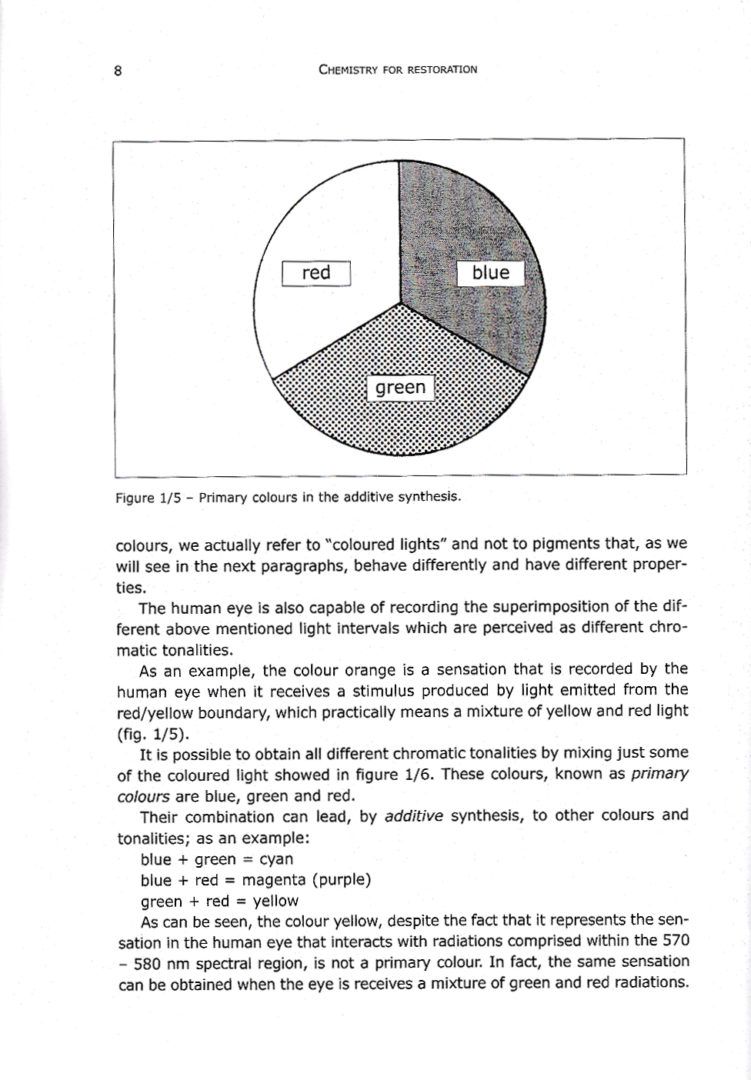
1.2.7 – Mixture of pigments
1.3– Chemical properties
1.4– Other pigments’ properties
1.5– Pigment classifications – Descriptive entries
1.5.1 – White pigments
1.5.2 – Violet pigments
1.5.3 – Blue pigments
1.5.4 – Green pigments
1.5.5 – Yellow pigments
1.5.6 – Red and orange pigments
1.5.7 – Brown pigments
1.5.8 – Black pigments
1.5.9 – Metals
Reference bibliography
Chapter two
BINDING MEDIA
2.1- Physical, optical and chemical properties of binding media
2.2 – The historical use of binding media
2.3 – Tempera
2.3.1 – Proteins
2.3.2 – Animal glues
2.3.3 – Egg
2.3.4 – Casein and milk
2.3.5 – Polysaccharides
2.3.6 – Polysaccharide temperas
2.3.6.1 – Vegetable gums
2.3.6.2 – Starches and dextrins
2.4 – Drying oils
2.5 – Mixed painting techniques
2.6 – Modern synthetic binders
Reference bibliography
Chapter three
SOLVENTS AND SOLUTIONS
3.1 – Introduction
3.2 – Solutions
3.3 – The solubilisation process
3.4 – The concept of solubility
3.5 – Solubilisation mechanisms
3.5.1 – Solubilisation of crystalline solids
3.5.2 – Solubilisation of non-crystalline solids
3.6 – Solvent power
3.7 – Other properties of solvents
3.7.1 – Vapour pressure, boiling point, volatility, retention
3.7.2 – Surface tension, wettability, surfactants, emulsifiers
3.7.3 – Viscosity of solvents and solutions
3.7.4 – Flammability, toxicity
Reference bibliography
Chapter four
CLEANING AGENTS AND SYSTEMS
4.1 – Classification and characteristics of most common solvents
4.1.1 – Hydrocarbons
4.1.2 – Halogenated hydrocarbons
4.1.3 – Alcohols
4.1.4 – Ethers
4.1.5 – Esters
4.1.6 – Ketones
4.1.7 – Aldehydes
4.1.8 – Ammonia and derivatives
4.1.9 – Other solvents
4.2 – Reactive solvents
4.3 – Selection of solvent
4.3.1 – Feller’s solubility test
4.4 – Enzymes
4.5 – Bacteria
4.6 – Ion exchange resins
4.7 – Laser cleaning
4.8 – Emulsions and surfactants
4.9 – Resin soaps
4.10 – Solvent-gels
Reference bibliography
Chapter five
VARNISHES, ADHESIVES, CONSOLIDANTS, FILLERS
5.1 – Introduction
5.2 – Natural resins
5.2.1 – Chemical and physical properties
5.2.2 – Classification of natural resins
5.2.3 – Oleoresins and balsams
5.2.4 – Resins
5.2.5 – Copals, amber and other fossil resins
5.2.6 – Resins from animals
5.3 – Synthetic resins
5.3.1 – Polyaddition resins
5.3.2 – Polycondensation resins
5.3.3 – Cellulose and cellulose derivatives
5.3.3.1 – Cellulose
5.3.3.2 – Cellulose derivatives
5.4 – Waxes
5.4.1 – Classification of waxes
5.5 – Varnishes and protective coatings
5.5.1 – General remarks
5.5.2 – Spirit varnishes: film-forming mechanism
5.5.3 – Properties of varnishes
5.5.3.1 – Protective functions
5.5.3.2 – Optical-aesthetic functions
5.5.4 – Varnish composition and other properties
5.6 – Adhesives, consolidants and fillers
5.6.1 – Adhesives
5.6.1.1 – Mechanisms of chemical adhesion
5.6.1.2 – Properties of adhesives
5.6.1.3 – Classes of adhesives
5.6.2 – Consolidants
5.6.2.1 – Mechanisms of “setting”
5.6.2.2 – Properties of consolidants
5.6.2.3 – Consolidants applied in restoration practice
5.6.3 – Fillers
5.6.3.1 – Mechanical properties
5.6.3.2 – Porosity
5.6.3.3 – Problems of reversibility
5.6.3.4 – Adhesive properties
5.6.3.5 – Hardening time
5.6.3.6 – Shrinkage
5.6.3.7 – Types of fillers
5.6.3.8 – Adhesives and fillers of mineral-origin (limes and cements)
Reference bibliography
PART II
BASICS OF CHEMISTRY
Chapter one
STRUCTURE OF MATTER AND INORGANIC CHEMISTRY
1.1 – The atomic structure of matter
1.2 – Chemical elements, compounds and mixtures
1.3 – Atomic structure
1.4 – Orbitals, electronegativity, chemical bonds
1.5 – Intermolecular bonds. Hydrogen bond
1.6 – Valence, oxidation number and oxidation state
1.7 – Aggregation states of matter
1.8 – Heterogeneous mixtures
1.9 – Chemical compounds and their nomenclature
1.10 – Oxides
1.11 – Acids and bases
1.12 – Measure of acidity – pH
1.13 – Strengths of acids and bases
1.14 – Salts
1.15 – Amphoteric behaviour
1.16 – Hydrolysis
Chapter two
CHEMICAL REACTIONS
2.1 – Concept of chemical reaction
2.2 – Exothermic and endothermic reactions
2.3 – Catalysis
2.4 – Types of chemical reactions
Chapter three
BASICS OF ORGANIC CHEMISTRY
3.1 – Organic compounds
3.2 – Classification of organic compounds
3.3 – Hydrocarbons, nomenclature and properties
3.4 – Isomerism
3.5 – Halogenated hydrocarbons
3.6 – Alcohols
3.7 – Ethers
3.8 – Aldehydes and ketones
3.9 – Carboxylic acids
3.10 – Esters
3.11 – Amines
3.12 – Macromolecules – Polymerization
Reference bibliography
Index
Recensisci per primo “Chemistry for Restoration. Painting and Restoration Materials” Annulla risposta
Devi effettuare l’accesso per pubblicare una recensione.













































Recensioni
Ancora non ci sono recensioni.